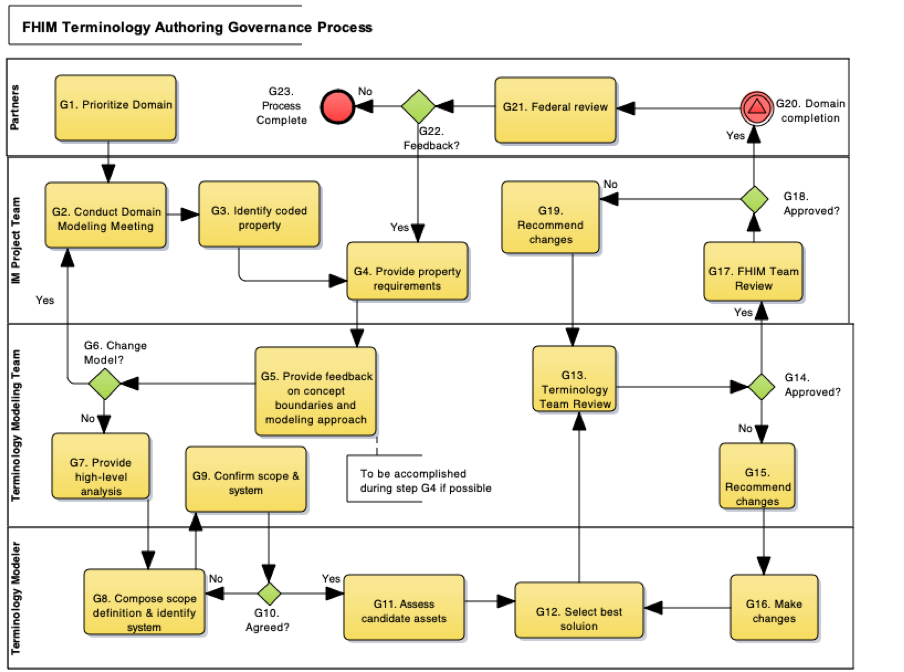
The process used to develop terminology was integrated with the process used to develop the information model. Separate meetings were held, but the terminology calls for a domain overlapped with the information modeling calls. This ensured that the processes were not disjoint, and terminology-driven questions could be addressed by domain experts. The objectives for these calls were threefold.
First, we clarified and confirmed the semantics of the target specifications. This often meant comparative analysis. Substance sensitivities, for instance, are classified by distinct axes (class of substance, such as drug or food, and class of reaction physiology, such as allergy and intolerance) in one specification, and they are pre-coordinated into a single classification in another.
Second, we identified implementable value sets for use in FHIM-supported interactions. Early on, this effort included some authoring work, but later devolved into identifying the extant specifications and documenting them in a common framework
And third, we ensured the FHIM data types and value set definitions supported the requirements of the client specifications.
The team authored a detailed process definition to support these activities, defining processes for the authoring, publication, and implementation of the identified assets. This process was also a key input into the HL7 Value Set.
Definition specification project
For a time, collaboration work on harmonization was conducted on the NLM’s VSAC Collaboration website. Later in the program, we focused our effort on identifying existing specifications. Early FHIM terminology content is published in the CDC’s PHIN VADS terminology server; later work is published in the NLM’s VSAC terminology server.