|
| AdverseEventReporting UML Documentation |
AdverseEventReporting
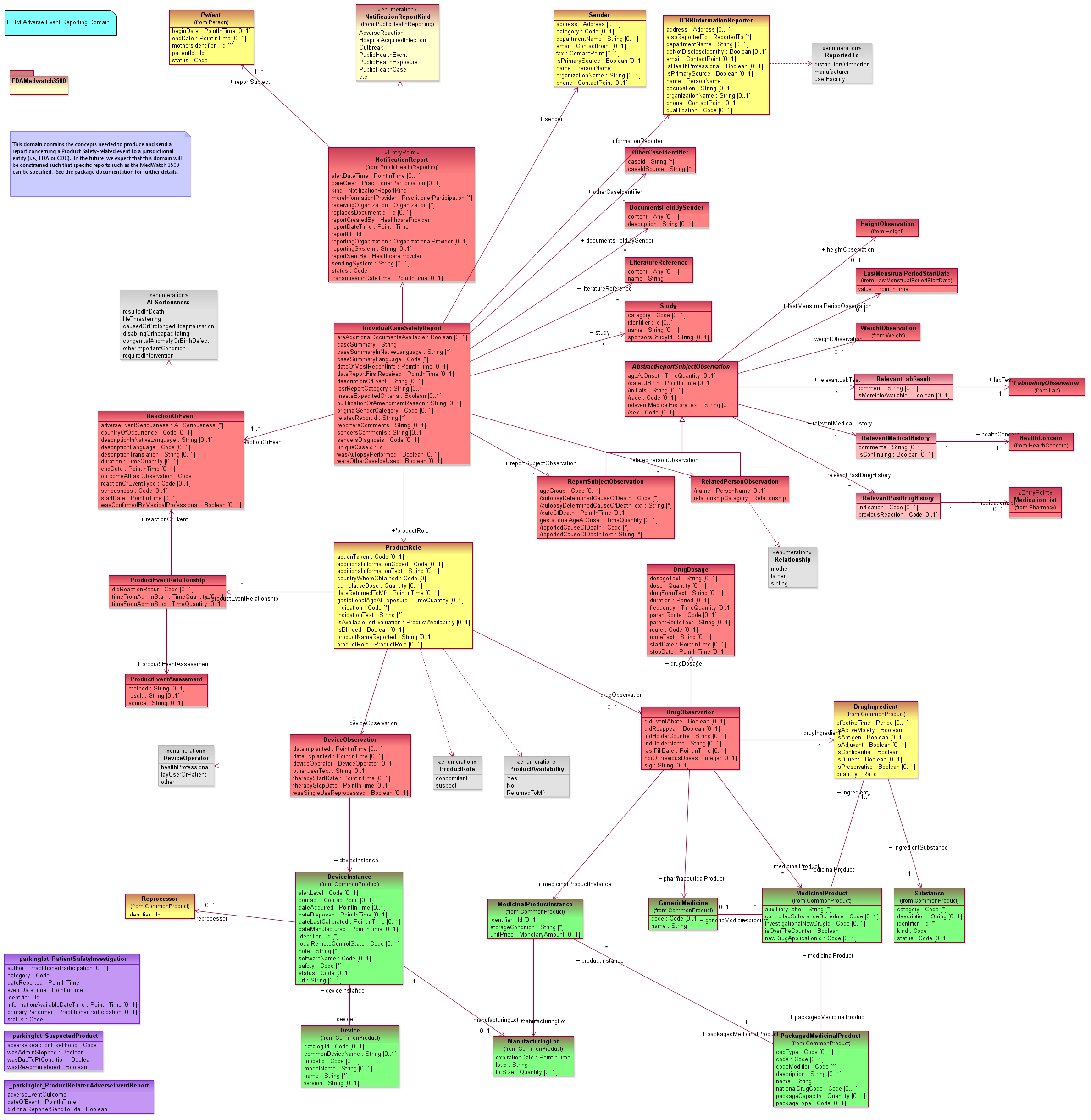
An adverse event is any untoward medical occurrence in a patient which does not necessarily have a causal relationship with any treatment they were undergoing, or an injury resulting from a patient's medical management rather than from the underlying condition itself.This domain contains those concepts needed to produce and send a report concerning an Adverse Event, especially a Product Safety-related event (which includes unexpected reactions to a medication) to a jurisdictional entity (i.e., FDA or CDC). This domain is closely aligned with the HL7 Individual Case Safety Report (ICSR) standard. The ICSR models the superset of concepts needed for the FDA Medwatch and VAERS reports, but does not include non-drug products (such as medical devices, cosmetics, or food supplements) and does not deal with non-human applications (e.g., veterinary medicine). We intend to add structures for these concepts in the future.Adverse Event Reporting is considered a kind of Public Health Report, so we expect a hierarchy from Public Health Report to Product-Related Adverse Events to specific kinds of reports such as the Medwatch reports. Other kinds of adverse events will likely also be reportable and will have other characteristics. For now, we are concentrating on the Product-Related Adverse Events. Eventually, this domain will be constrained such that specific reports such as the MedWatch 3500 can be specified. At that time separate sub-packages will be created for various types of reports such as the MedWatch 3500 and the VAERS-1.
 |
Properties:
| Alias | |
| Keywords | |
| Name | AdverseEventReporting |
| Name Expression | |
| Namespace | FHIM |
| Nesting Package | FHIM |
| Owned Template Signature | |
| Owner | FHIM |
| Owning Template Parameter | |
| Qualified Name | FHIM::AdverseEventReporting |
| Stereotype | |
| Template Parameter | |
| Visibility | Public |
|
| AdverseEventReporting UML Documentation |

